Bond energies and bond lengths. Web the amount of energy required to break all covalent bonds of the same type in one mole of a compound in a gaseous state is called bond energy. This is because as we go down the group, the atomic radius increases. Lewis diagram of formaldehyde (ch₂o) Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a triple bond).
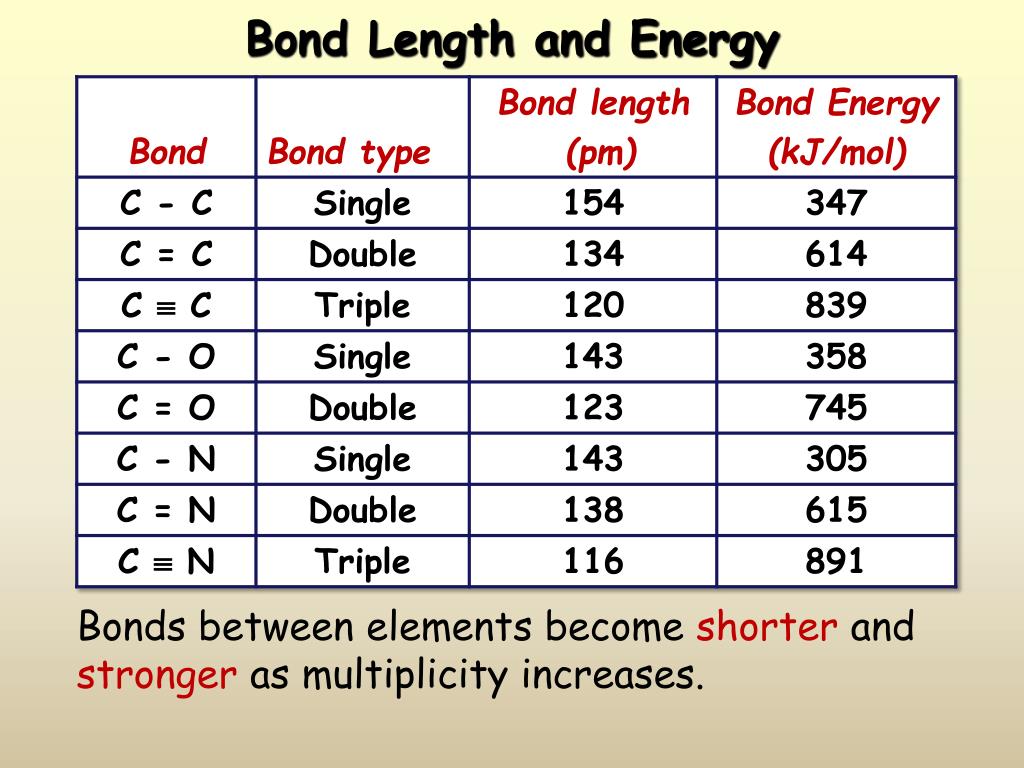
Web bond length is defined as the distance between the centers of two covalently bonded atoms. Web from this graph, we can determine the equilibrium bond length (the internuclear distance at the potential energy minimum) and the bond energy (the energy required to separate the two atoms). Before we go into the details explaining the bong lengths and bond strengths in organic chemistry, let’s put a small summary for these two properties right from the beginning as it stays relevant for all types of bonds we are going to talk about. Bond order is the number of electron pairs that hold two atoms together. Web below is a table of average bond lengths.
The higher the bond order, the stronger the pull between the two atoms and the shorter the bond length. Bond order is the number of electron pairs that hold two atoms together. Lewis diagram of formaldehyde (ch₂o) Bond length and bond strength. Web in molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule.
It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Web bond length is defined as the distance between the centers of two covalently bonded atoms. Single and multiple covalent bonds. Bond energies and bond lengths. As we go across a period we see bond length decreases. Web below is a table of average bond lengths. A shorter bond length has higher bond energy. The higher the bond order, the stronger the pull between the two atoms and the shorter the bond length. Browse videos, articles, and exercises by topic. Web from this graph, we can determine the equilibrium bond length (the internuclear distance at the potential energy minimum) and the bond energy (the energy required to separate the two atoms). Web the amount of energy required to break all covalent bonds of the same type in one mole of a compound in a gaseous state is called bond energy. Bond length and bond strength. 8.9.1), where the bottom of the well represents the equilibrium position of the oscillating atoms, which. The bond energy is inversely proportional to the bond length. This is because as we go down the group, the atomic radius increases.
Bond Order Is The Number Of Electron Pairs That Hold Two Atoms Together.
The length of the bond is determined by the number of bonded electrons (the bond order). A shorter bond length has higher bond energy. Lewis diagram of formaldehyde (ch₂o) Web below is a table of average bond lengths.
Web From This Graph, We Can Determine The Equilibrium Bond Length (The Internuclear Distance At The Potential Energy Minimum) And The Bond Energy (The Energy Required To Separate The Two Atoms).
Web bond length is defined as the distance between the centers of two covalently bonded atoms. A bond is not static but dynamic with the atoms undergoing the attractive and repulsive forces as described in the potential well (fig. Predicting bond type (metals vs. Before we go into the details explaining the bong lengths and bond strengths in organic chemistry, let’s put a small summary for these two properties right from the beginning as it stays relevant for all types of bonds we are going to talk about.
This Is Because As We Go Down The Group, The Atomic Radius Increases.
It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Bond energies and bond lengths. This unit is part of the chemistry library. The bond energy is inversely proportional to the bond length.
Web In Molecular Geometry, Bond Length Or Bond Distance Is Defined As The Average Distance Between Nuclei Of Two Bonded Atoms In A Molecule.
Bond length and bond strength. Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a triple bond). Web the amount of energy required to break all covalent bonds of the same type in one mole of a compound in a gaseous state is called bond energy. As we go across a period we see bond length decreases.
![Bond lengths [Å] and angles [°] for 4c Download Scientific Diagram](https://www.researchgate.net/publication/333256073/figure/download/tbl2/AS:761180935159811@1558491085331/Bond-lengths-A-and-angles-for-4c.png)

.PNG)