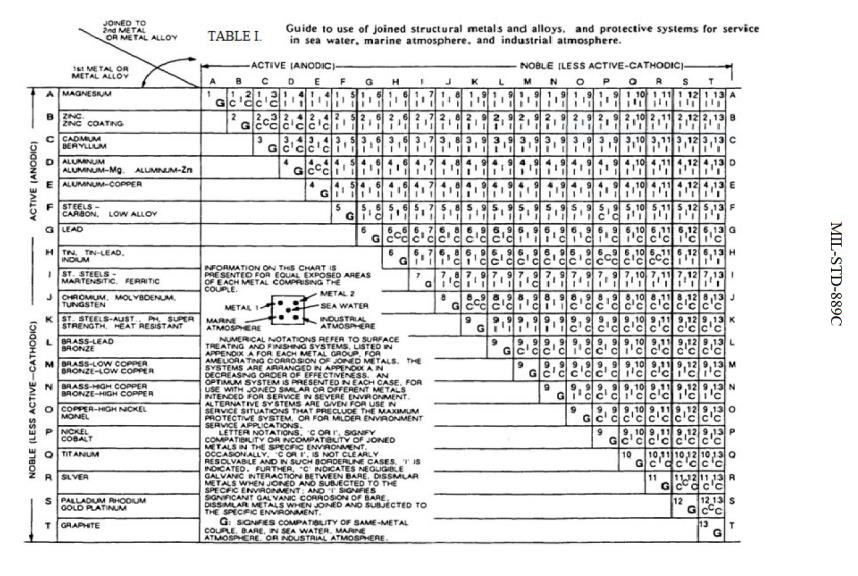
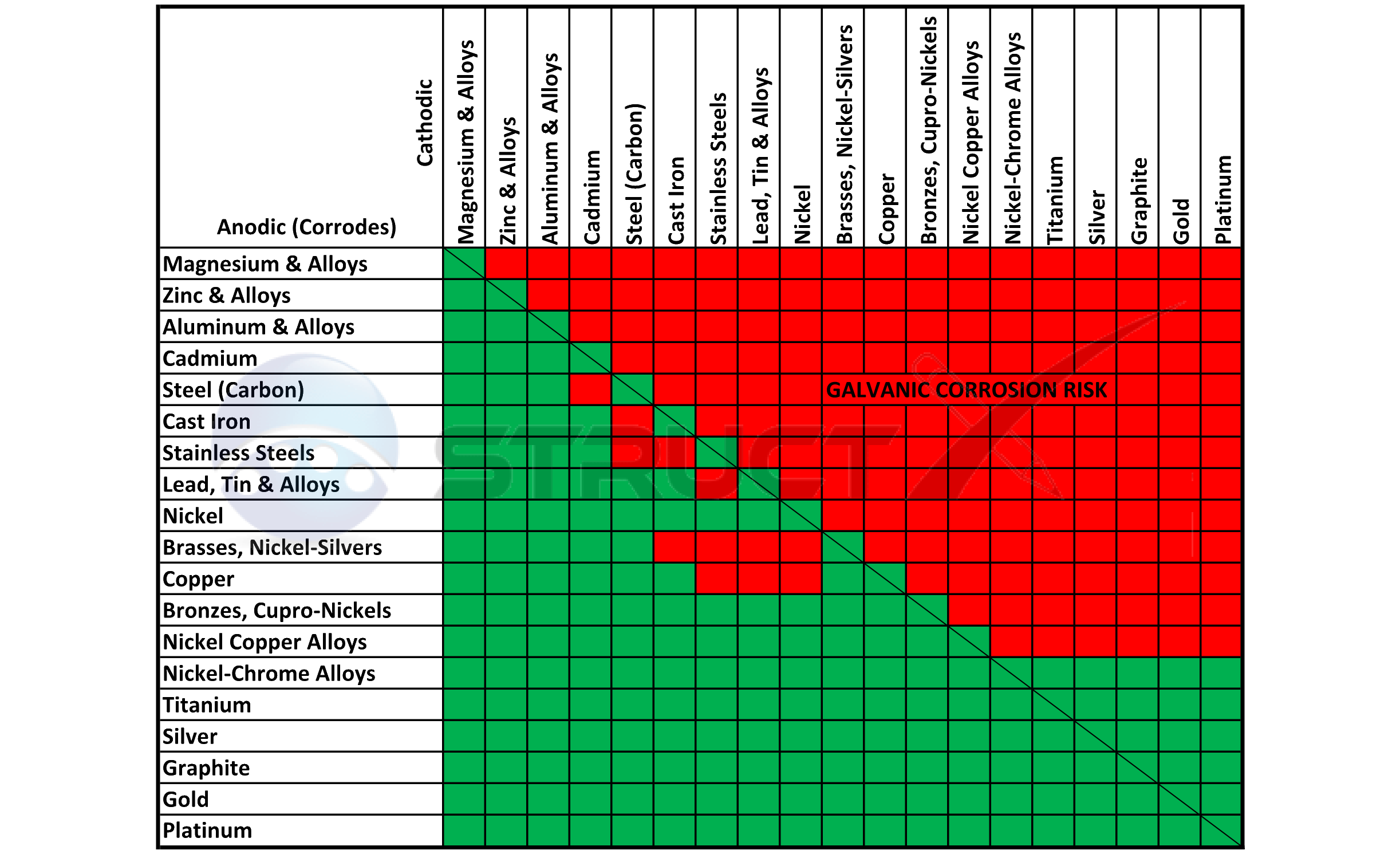
Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal. For any combination of dissimilar metals, the metal with the lower number will act as an anode and will corrode. Web below is a galvanic reaction chart for dissimilar metals. Web below, we delve into dissimilar metal corrosion, how and why it occurs, and tips for avoiding it to prevent accidents and damaging project delays. During this process, corrosion occurs on the anode, whereas.
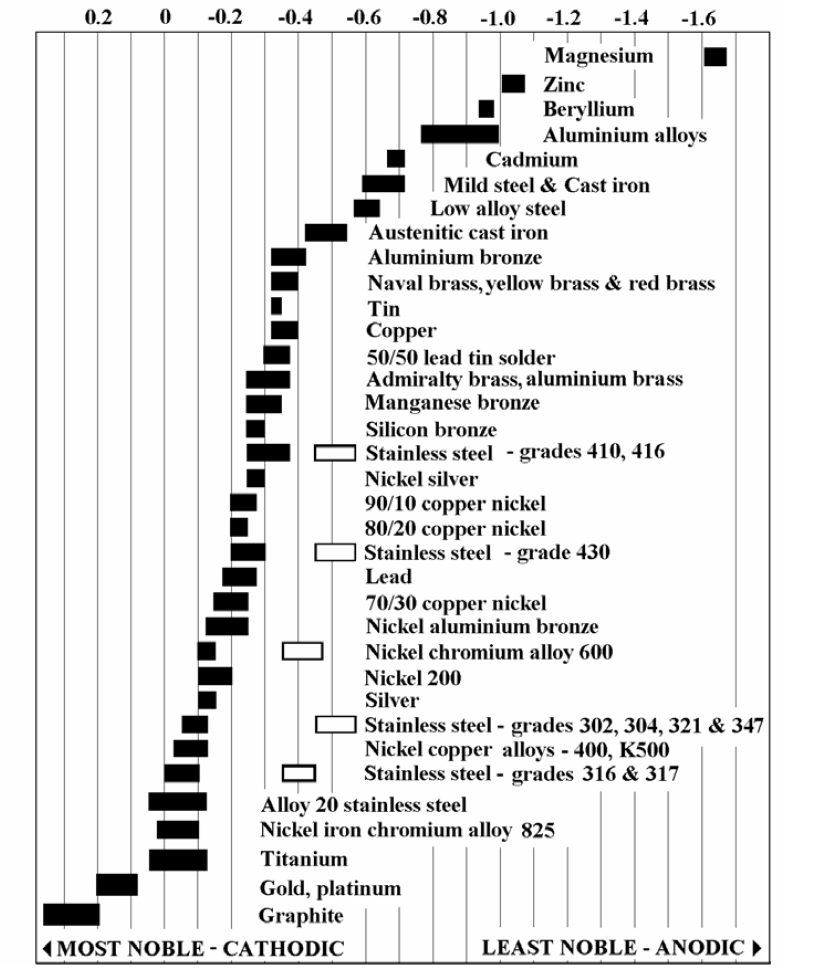
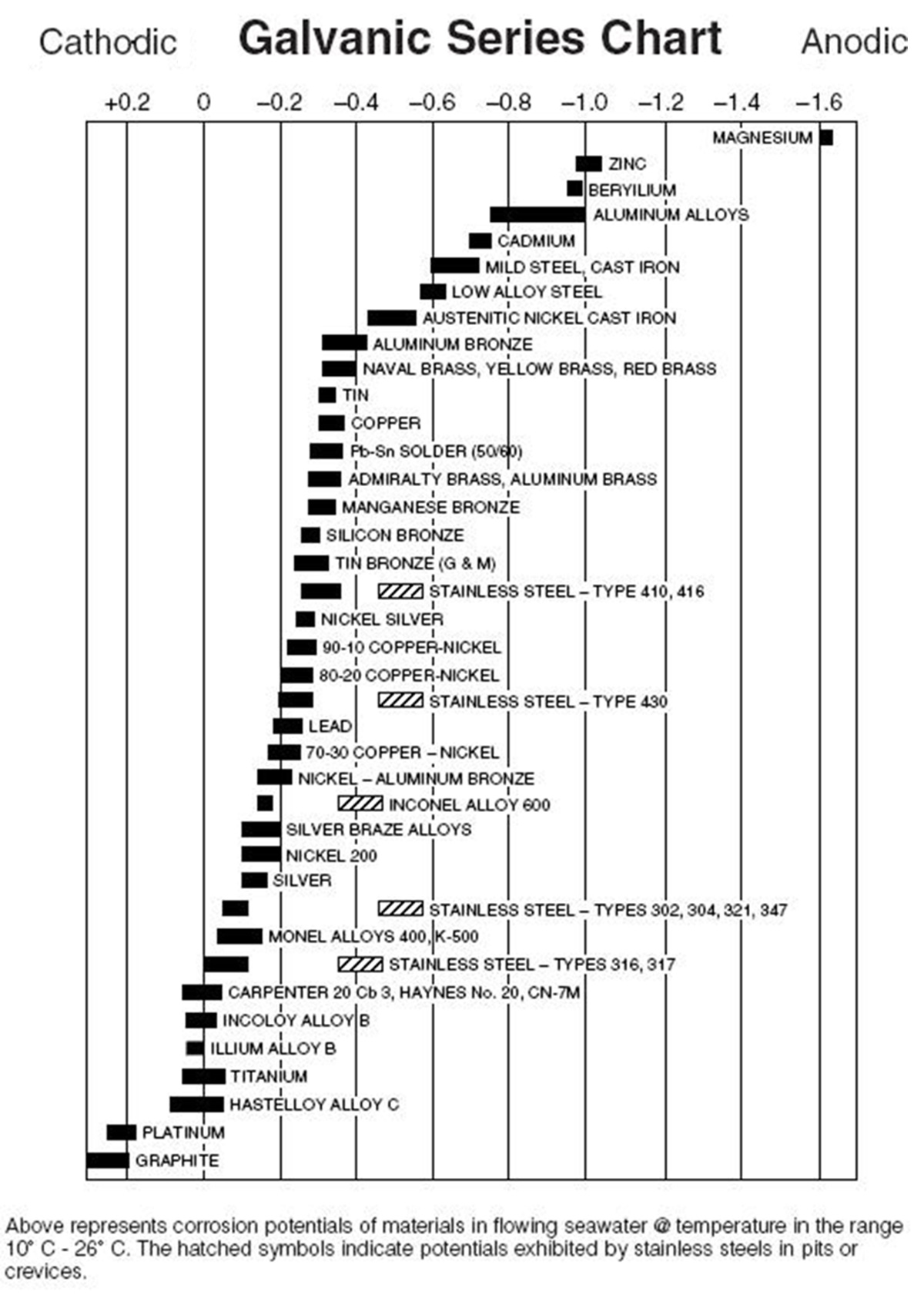
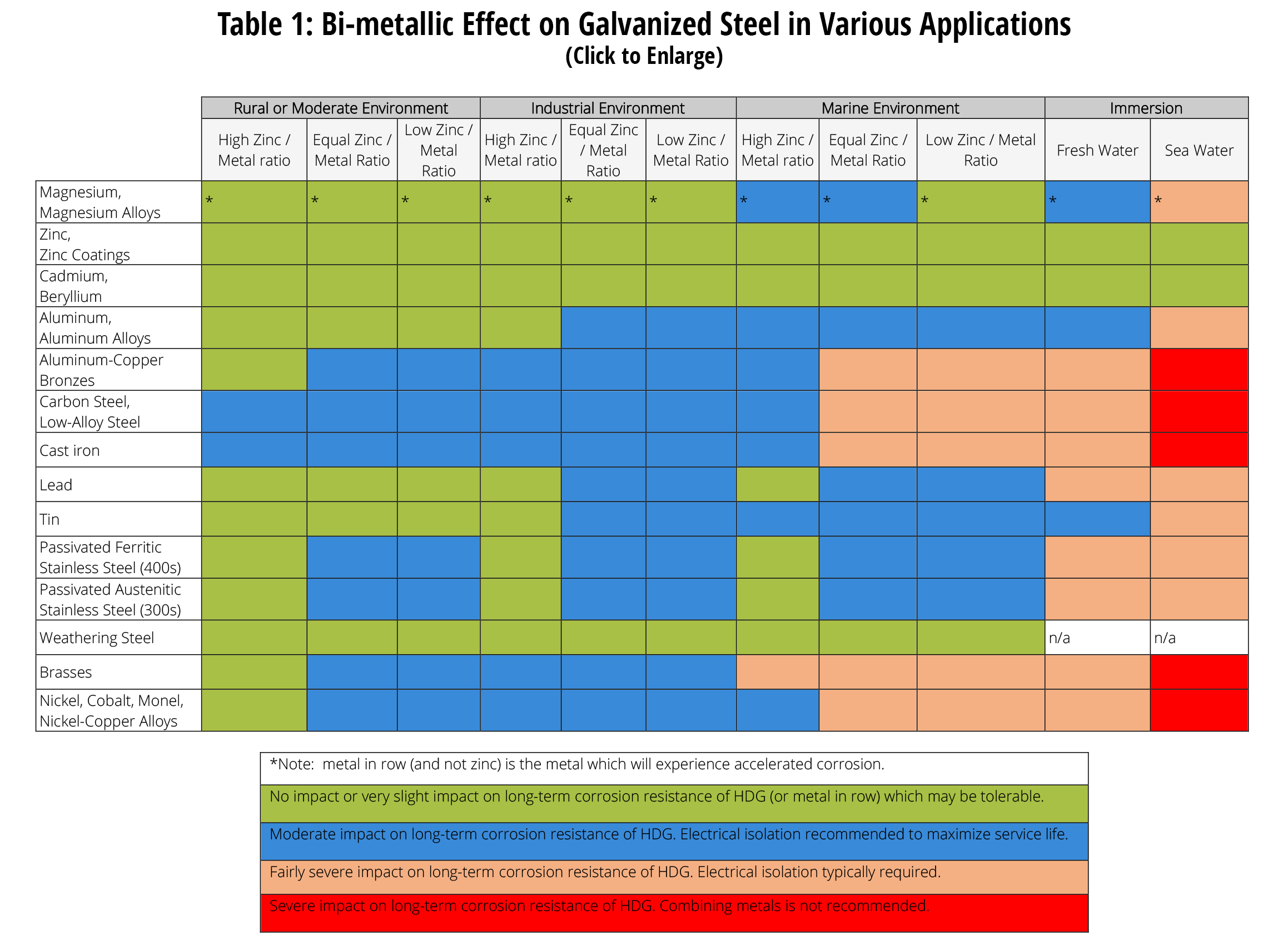
Web when design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. Web galvanic corrosion occurs when two dissimilar metals are in contact electrically in the presence of an electrolyte. It may also take place with one metal with. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Web the susceptibility of different base metals to corrosion while in contact depends upon the difference between the contact potentials or the electromotive voltages of the metals.
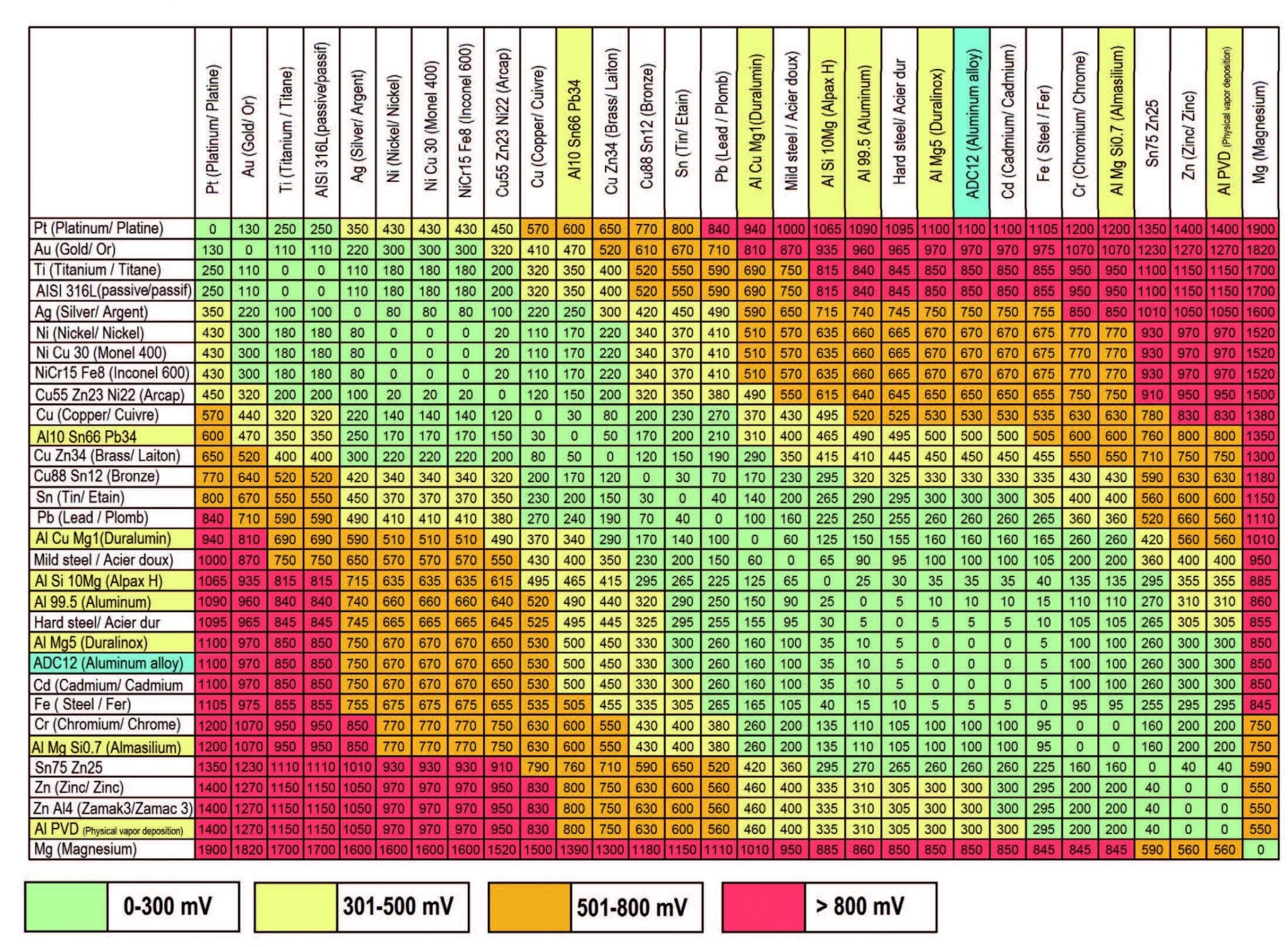
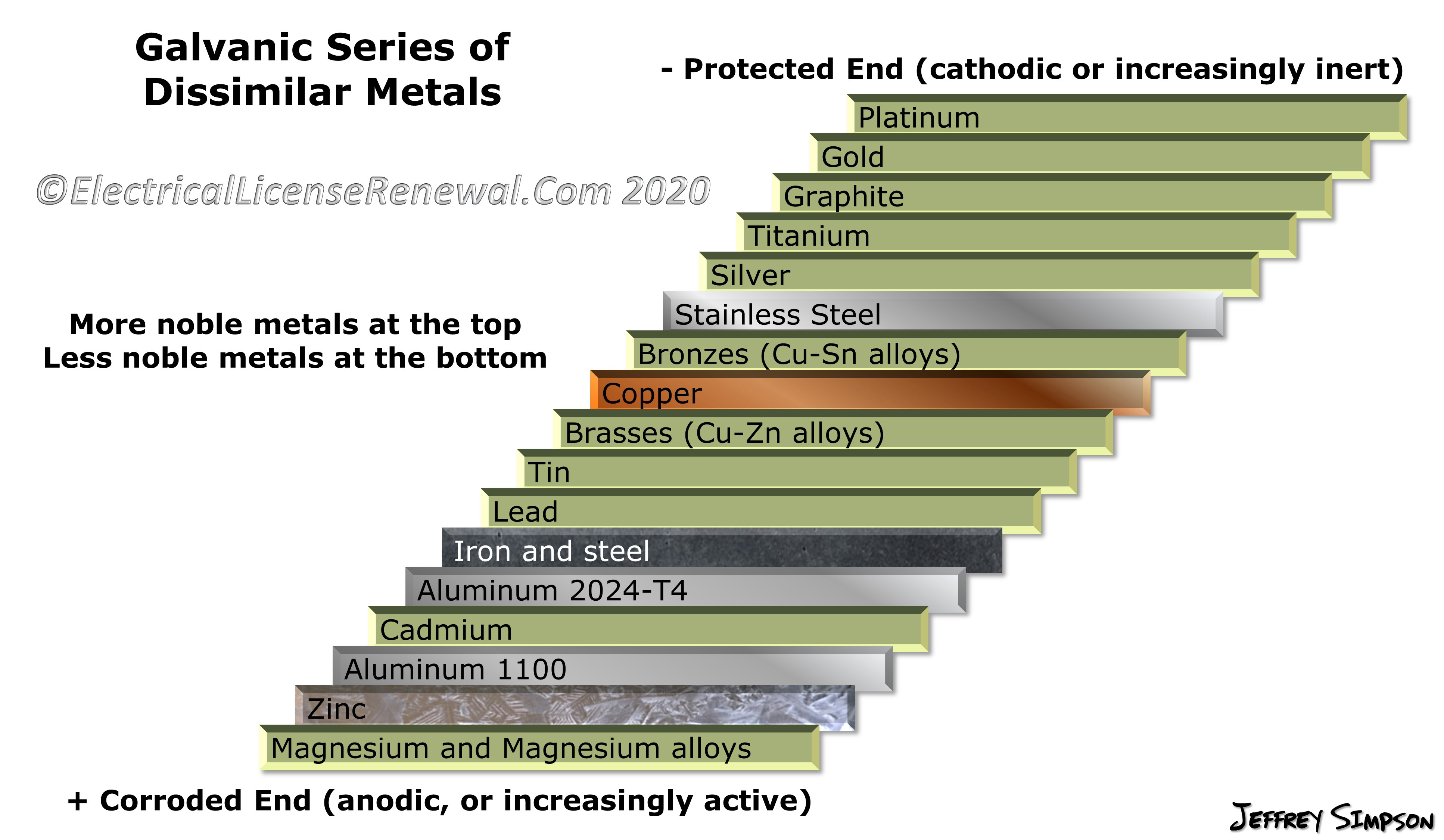
Web galvanic series, or nobility chart for dissimilar metals. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. So, for example, choosing zinc on zinc would have the lowest risk for. Web find out how dissimilar metals will corrode when placed against each other in an assembly using this chart. Web galvanic corrosion occurs when two dissimilar metals with different potentials are placed in electrical contact in an electrolyte.
This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. Web galvanic series, or nobility chart for dissimilar metals. Use this chart to avoid galvanic corrosion in seawater when different metals come in to contact. The chart shows the galvanic corrosion potential of various metals. Web galvanic corrosion typically attacks junction areas of dissimilar metals and occurs when the following three conditions are met. If two different metals are placed in electrical contact and bridged by an electrolyte, a current figure 1: Web this article examines how dissimilar metals can lead to galvanic corrosion. This phenomenon is named after italian ph… So, for example, choosing zinc on zinc would have the lowest risk for. Web below, we delve into dissimilar metal corrosion, how and why it occurs, and tips for avoiding it to prevent accidents and damaging project delays. Web galvanic corrosion occurs when two dissimilar metals are in contact electrically in the presence of an electrolyte. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. During this process, corrosion occurs on the anode, whereas. It includes a chart that shows how different plating materials react to one another with.
Galvanic Corrosion (Also Called Bimetallic Corrosion Or Dissimilar Metal Corrosion) Is An Electrochemical Process In Which One Metal Corrodes Preferentially When It Is In Electrical Contact With Another, In The Presence Of An Electrolyte.
The chart shows the galvanic corrosion potential of various metals. For any combination of dissimilar metals, the metal with the lower number will act as an anode and will corrode. Web the susceptibility of different base metals to corrosion while in contact depends upon the difference between the contact potentials or the electromotive voltages of the metals. Use this chart to avoid galvanic corrosion in seawater when different metals come in to contact.
This Phenomenon Is Named After Italian Ph…
Find a table of anodic index values for. Web below, we give a brief overview of galvanic corrosion and provide a galvanic corrosion chart to help fabricators and machinists avoid using the wrong metal. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. If two different metals are placed in electrical contact and bridged by an electrolyte, a current figure 1:
It May Also Take Place With One Metal With.
Web learn how to prevent galvanic corrosion (dissimilar metal corrosion) by understanding the three conditions that must exist for it to occur. During this process, corrosion occurs on the anode, whereas. Web galvanic corrosion occurs when two dissimilar metals are in contact electrically in the presence of an electrolyte. So, for example, choosing zinc on zinc would have the lowest risk for.
Web Find Out How Dissimilar Metals Will Corrode When Placed Against Each Other In An Assembly Using This Chart.
Web when design requires that dissimilar metals come in contact, galvanic compatibility can be managed by finishes and plating which protects the base materials from corrosion. Web electrolytic corrosion (electrolysis) occurs when dissimilar metals are in contact in the presence of an electrolyte, such as water (moisture) containing very small amounts of. It includes a chart that shows how different plating materials react to one another with. Web below, we delve into dissimilar metal corrosion, how and why it occurs, and tips for avoiding it to prevent accidents and damaging project delays.